Probing Microbes
Gira Bhabha
Johns Hopkins University
Published September 30, 2025
For three years, tuberculosis (TB) was replaced by COVID-19 as the planet's deadliest infectious disease, but in 2023 TB's causative agent, mycobacterium tuberculosis, regained its rank as the world's most lethal microbe.
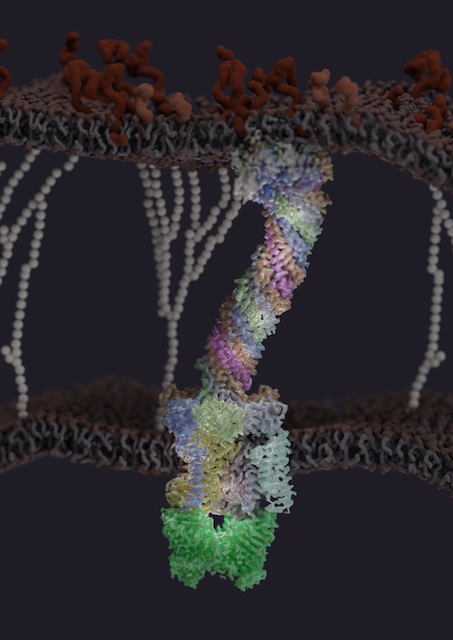
The same year, Gira Bhabha and her colleagues revealed the first structure of a molecular component that allows the bacterium to thrive in people's cells and cause disease (Nature, 2023). The protein complex spans the entire cell envelope of the bacterium and may be a potentially effective drug target for TB.
mTB kills more than 1 million people every year. It is preventable and treatable, but many people have no access to the necessary medical care, and the treatment regimens are extensive, with a high drop-out rate that leads to multi-drug-resistant disease.

After it infects people, the TB mycobacterium can linger inside some cells, typically macrophages in the lung, thanks to a notoriously tough barrier shielding it from antibiotics and the immune system. But the bacterium's protective cell envelope also walls off the nourishment only the host can provide. That's where the protein complex comes in.
The proteins are called mammalian cell entry (MCE) proteins. They belong to a protein family discovered decades earlier as crucial, somehow, to TB virulence.
Recent evidence from other scientists suggested a hypothesis. The protein family "might be important for stealing nutrients from the host cell for the survival of the bacterium," says Bhabha. "But we had very little information on it. What does this family of proteins look like, what does it do, and how?"
The molecular structure from Bhabha and her colleagues provided the first insights into how this virulence factor might work. Nearly a dozen MCE proteins assemble to form a kind of feeding tube through the bacterial wall to scavenge nutrients.
"It's almost like a straw," Bhabha says. "The nutrient, or the substrate, would be able to travel all the way down the tube, across this pretty gnarly cell envelope, and then end up inside the bacterium."
The study, led by postdoctoral fellow James Chen, serves as a starting framework to understand the MCE transport system. Bhabha and her colleagues are following up to explore shape changes at the atomic level that allow the feeding tube to function, identify other protein partners, and examine the interface of the tube with the outer membrane.
It takes a whopping 24 liters of cell culture to isolate enough material for cryo-electron microscopy (cryo-EM). They developed the structural model by combining mass-spectrometry data, structure predictions from the machine-learning platform AlphaFold2 and extensive cryo-EM computational analysis.
"It's actually not something that you would look at the raw data from the cryo-EM and think, 'Oh, that's beautiful,'" Bhabha says. "It looks quite unappealing, but then, because of all the advanced data processing tools that so many of our colleagues in the field have developed, we were able to get to the point of resolving the entire complex. We really appreciate that with SBGrid, we can readily have access to so many of these tools."
Bhabha runs a combined laboratory with microbiologist and structural biologist Damian Ekiert at Johns Hopkins University in Baltimore. Lab members share projects, equipment, reagents, group meetings, lab retreats and outings. The research broadly focuses on how protein structure, function and dynamics work together in biological systems related to microbes, both prokaryotic and eukaryotic.
"Mixing of different perspectives, ideas and skills usually results in elevating our grasp of any system," notes the Bhabha+Ekiert labs website, "so collaboration and communication within the lab and with others outside the lab is highly encouraged."
From Sequence to Structure
Bhabha grew up in Mumbai, India. At the time, high school sophomores took a national test to sort them into one of several specialized educational tracks in their junior and senior years. Her test results gave her the option of studying humanities or science, and classes from the two could not be mixed and matched. Although she had a strong interest in both, she studied humanities and did not take any science classes.
She rediscovered science in the core undergraduate curriculum at University of Chicago. She had intended to major in psychology and English literature, but she switched to a pre-med track with dreams of going into veterinary medicine.
Her genetics professor asked if she ever considered work in a laboratory. "I hadn't really thought of science as a career before, so I thought, 'that sounds fun,'" she says. And she found it exciting. Her lab work investigated a fruit-fly model for muscular dystrophy. In the back of her mind, her graduate school plans enlarged to pursuing dual degrees, DVM and PhD.
Along the way, she spent a lot of time peering at the fine details of cells and tissues with optical and confocal microscopy, which raised tantalizing questions about the smaller unseen molecular changes. She was struck by the link between sequence and structure, but she was also fascinated with how very different genetic sequences could yield similar three-dimensional protein structures.
"I really wanted to know more at the level of proteins," she says. "I like thinking about proteins and being able to really just see what you're studying.
That led her in 2006 to a PhD in structural biology in Peter Wright's lab at Scripps Research in La Jolla, Calif. "I got more fascinated with protein dynamics," she says. "We were more used to thinking of protein structures at the time as static. But you often can't just look at one snapshot and understand how something works."
Bhabha worked with a model enzyme combining X-ray crystallography and nuclear magnetic resonance (NMR) imaging to characterize the dynamics at different timescales. She compares the concept to the famous horse-in-motion photo series in the late 1800s that documented how the legs moved at high speed.
"It's exactly the same thing at a molecular level, with snapshots of the proteins in action to see how they work," Bhabha says. She applied her genetics tactics to "knock out" protein dynamics in the enzyme by introducing point mutations that had a minimal effect on the structure but a major defect in the molecular movement. "Together with collaborators, we were able to put together one of the first descriptions of the impact of dynamics on the catalysis of the enzyme," she says.
For her postdoctoral fellowship, she wanted to understand how proteins work together in large macromolecular complexes within cells. She moved to University of California, San Francisco, to work in the lab of Ron Vale to study the large motor protein dynein and to learn cryo-EM from her co-mentor, Yifan Cheng. She and her colleagues wanted to understand how the protein walks along microtubule tracks to transport cargo. Combining single particle cryo-EM with single molecule microscopy and biochemistry, they were able to arrive at a model for how conformational changes in the dynein motor domain are coupled to its ATP hydrolysis cycle.
In 2017, she joined the research faculty at the New York University School of Medicine, where she and Ekiert moved to start independent labs. They had collaborated on separate projects since graduate school and became life partners along the way. In New York, from the very beginning, they integrated their groups together around the themes of the structural mechanisms and cell biology of microbes, using a range of tools and techniques to image across scales from atomic to cellular.
Harpoon invasion
In 2024, they moved their combined labs to Johns Hopkins. The MCE family of proteins and their role in bacterial physiology and virulence is one of the labs' major research programs funded by the National Institutes of Health. Bhabha cites it as a structure-driven example of their work. Another research program focuses on microsporidia and bridges cell and structural biology to understand how, exactly, it infects the host cell. "It has a very unique way of doing that," Bhabha says.
Microsporidia are single-celled parasites with more than 1700 species that can infect almost any insect or animal on the planet. They can result in fatal illnesses in immune-compromised patients, such as in people with organ transplants or AIDS. They have been implicated in honey-bee colony collapse, in decreased yield in farmed seafood such as shrimp, and in other agriculturally important animals.
The tiny parasites form spores that resemble miniature eggs. But coiled inside the spore, is a very long tube that springs into action. The prevailing hypothesis is that microsporidia use the polar tube to enter a new cell, she says. The organelle is not found in other cells and eukaryotes.
"When it's triggered, it shoots that tube out like a harpoon, and then that harpoon impales the host cell," Bhabha says. "Then through the hollow tube that forms, infectious material is transferred from the pathogen into the host cell. It's very fast. The tube fires in a few 100 milliseconds, and the whole infection process is over in just a second or two."
People may pick up the infection from contaminated drinking water or food. Once in the intestine, an unknown trigger will make the microsporidia fire their harpoons, which would hit and infect intestinal cells.
So far, using cryo-electron tomography, the researchers have found evenly spaced filaments of proteins in two layers that likely form the skeleton of the tube, surrounded by a membrane and filled with a dense core of unknown composition (PNAS, 2025). They are taking their structural studies to higher resolution and working in the structural cell biology realm to evaluate interactions of the pathogen with the host cell.

In her life outside the lab, Bhabha tries to unwind with yoga, hikes and creative vegetarian meals. She parents two rescue dogs, a pit bull mix and a pointer, both of which are featured on the lab's web pages and retreat photos.
By Carol Cruzan Morton

































































































