Mind and Muscle
Ryan Hibbs
University of California, San Diego
Published March 28, 2025
From ancient Greece to modern day, people have often considered activities of the mind and muscle as fundamentally separate, even opposite, pursuits. Consider the contrasting stereotypes of a nerdy brainiac and a brawny body builder.
But at the molecular level, the function of synapse and sinew rely on a shared family of ion channels, the tiny pores in a cell’s membrane that enable split-second signaling in brain and body.
Ryan Hibbs at the University of California, San Diego, studies the structure and function of the ion channels to figure out how they behave in health, misfire in disease, and interact with drugs.
The lab mostly divides its attention between two types of ion channels. One type, best known in muscle, enables a newborn calf to stand up on shaky legs as soon as 15 minutes after birth, they have reported. Another type covertly helps epilepsy drugs combat seizures in people’s brains.
Both types function as neurotransmitter receptors. Variations of the muscle type are found in the brain and underpin nicotine addiction. The other type governs about a third of the connections between neurons in the brain and is the target of general anesthetics and anxiety medications, as well as drugs of abuse.
Recent collaborations have expanded the lab’s portfolio to new families of sensory receptors in octopus and squid. And the team has extended their ion channel work to autoimmune disease processes.
“I don’t have a specific passion and goal for my life of trying to solve some specific huge problem,” Hibbs says. “I just generally love neuropharmacology. I like puzzles.”
Snakebite Science
For muscles to spring into action, such as in breathing, blinking or body building, nearby neurons release acetylcholine, which binds to a receptor. Like others who study the receptor, the Hibbs lab grew its own proteins in cell cultures. But, they wondered, how much did these lab-made proteins have in common with real life?
In a recent project in the lab, they turned to fetal bovine tissue rescued from slaughterhouses. “We had read that the amount of the receptor is higher earlier in development,” Hibbs says. “And that makes sense, because the connections between the central nervous system and the muscle haven’t been wired up yet. When it gets wired up, synapses form at the junctions, and receptors that don’t have those synapses get removed. But before the junctions form, the receptors are all over the surface.”
Postdoctoral fellow Huanhuan Li took on the heavy lifting of purifying the receptor. He began with nearly 5 pounds of beef. To pull the receptor from the muscle tissue, he turned to another real-life source: Snake venom.
“It’s how these snake venoms work,” Hibbs says. “They cause paralysis by binding to the receptor at the same site where the neurotransmitter acetylcholine binds.”
The laborious purifications took a week, compared to the usual day or two, but it yielded the highest resolution structures to date from the lab (Nature, 2024). And there was a big surprise.
They found as much of the adult form of the receptor as the fetal form. In humans, the fetal form predominates until birth, when the adult form replaces them, Hibbs says. But when it comes to this receptor, cows grow up faster.
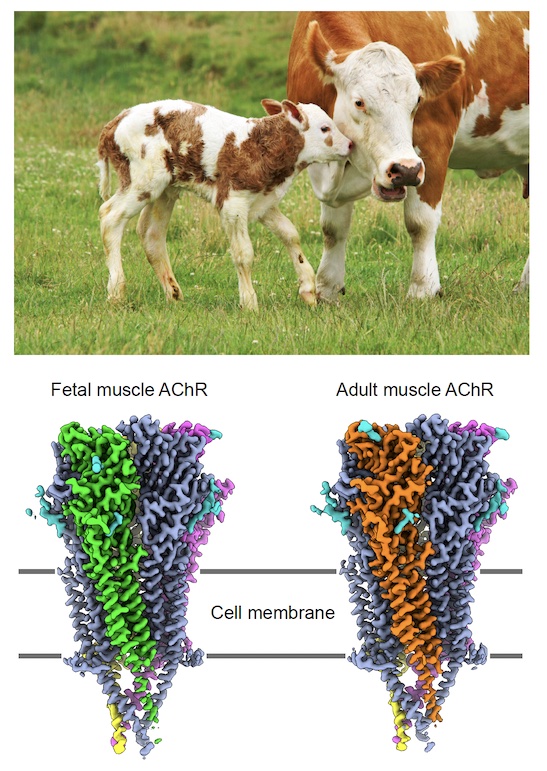
The fetal and adult forms differ by only one of the five subunits that circle the channel. The presence of both forms in the same cryo-electron microscopy (cryo-EM) sample allowed the team to explore how the structural difference between them underlies their different tasks.
The fetal form creates synapses at neuromuscular junctions, establishing organized electrical connections between the muscle and the spinal cord, while the adult form optimizes muscle contraction at synapses.
Hidden Epilepsy Drug Action
Meanwhile, for a new project on GABAA receptors, they were able to study brain tissue of people with epilepsy who underwent surgery to control their symptoms. The two forms of acetylcholine receptors in muscles may be well-defined, Hibbs notes, but much debate has swirled about the many possible variations of GABAA receptors found in our brains.
“We know what the subunits are,” Hibbs says. “We don’t know how many of them are in each receptor type, nor the order they’re in. We have good ideas about some of them. People argue about it, but it hadn’t really been conclusively shown.”
They consulted a neurosurgeon, who saved the resected tissue from 81 patients seeking relief from their symptoms, with each patient’s permission. In analyzing the samples, postdoctoral fellow Jia Zhou and her co-authors found 12 different structural variations of GABAA receptors, a challenging sorting task by cryo-EM.
“There was a surprise here as well,” Hibbs notes. They detected unexplained signals in cryo-EM density map of experimental structural information. Once more, they consulted the neurosurgeon. Many patients were taking drugs commonly prescribed for epilepsy, but the predominant antiepileptic drugs were not known to act on GABAA receptors.
Using electrophysiology and receptors purified from cell lines, Zhou and her coauthors tested drugs on GABAA receptors and mapped the structures. Then they went back to the native brain tissue data. The mystery signal turned out to be consistent with a previously unknown off-target modulator of GABAA receptors and could be contributing to its therapeutic efficacy, the researchers concluded. (Nature, 2025).
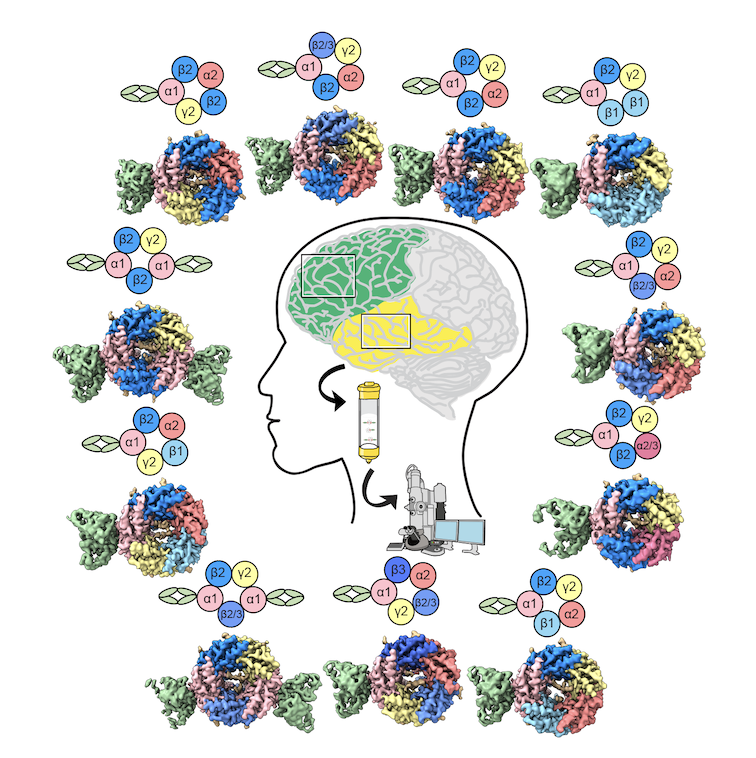
“That was really exciting to discover and another example of how when you go into tissue, whether it’s fetal calf tissue or human brain tissue from epilepsy surgeries, you find things you weren’t even looking for,” Hibbs says. “We want to keep doing this kind of thing.”
The Professional Journey
Hibbs grew up in Corvallis, Oregon, the son of a forestry professor (father) at Oregon State University (OSU) and special education teacher (mother). Now, as an outdoor enthusiast himself, Hibbs appreciates that his father picked a science job that took him outside for work. But in his first summer job in his early teens, Hibbs did not feel the same.
“I worked a series of — for me — awful summer jobs,” he says. “I’m small, and I was working on a farm and throwing hay bales around and stacking them. And I am just not cut out for that.”
Hibbs’ occupational satisfaction perked up after a high school biology class. The teacher had secured molecular biology kits. “We could extract DNA from cells … and actually look at DNA,” he says. “That was the most incredible thing. That was my first real spark of interest in science.” His dad connected Hibbs to an OSU research lab where he spent the next few summers working on plant genetics.
Hibbs attended Whitman College, a small liberal arts school in Washington State. He gravitated toward biochemistry. “I found that coursework really tickled my fancy,” he says. “I liked understanding proteins. ‘What are proteins doing? How do they catalyze reactions, enzymes and receptors? How do they signal? How do they take one molecule and turn it into another, and then also, how do drugs act on proteins to change their functions?’”
He switched summer lab jobs midway through college, when a classmate referred Hibbs to a neuropharmacology lab in Reno, Nevada. There, he worked on drug mechanisms in a uterine smooth muscle system and found an intellectual sweet spot in neuropharmacology.
He graduated with uncertainty about the next steps. His graduate school application was rejected. He cycled through a summer research position in a UCSD lab. In the fall he landed a job in a biotech company.
“It was all the things I love about academic research, which include getting to work with really smart people from all over the world to creatively solve hard problems,” he says. When the next round of graduate school applications came due, his biotech colleagues encouraged him.
He applied to UCSD, equally enchanted by the quality of the research and the geographic proximity to ocean, mountains and desert, along with the mild weather to support multiple outdoor recreation pursuits. And he got in.
For his graduate studies, he chose a neuropharmacology lab, which introduced him to the acetylcholine receptor, its neurotransmitter trigger and the lightning-fast enzyme that terminates the signal.
His timing was good. He joined the lab just after a Dutch group published the first robust structural information for the soluble portion of the receptor that sticks out of the membrane. The Dutch team had figured out that the soluble portion was made in high quantities by snails and other mollusks.
Hibbs used that structural foundation to explore the dynamics of the receptor and how drugs affect it. Another student established X-ray structural biology in the lab, and Hibbs embraced crystallography. He ended his doctoral studies with four first-author papers, including a crystal structure of the snail protein bound with various compounds.
“That gave me some confidence that structural biology wasn’t too risky to take on, and it revealed to me the power of structural biology to understand molecular interactions and drug mechanism,” he says.
Mastering Membrane Proteins
The next step was clear to Hibbs. “I saw that the field of acetylcholine receptor pharmacology was stuck because we had no structural information, at least at high resolution, of the full-length receptor with the ion channel.”
He did his postdoctoral work at one of the few labs having regular success with membrane protein crystallography, the only high-resolution technique at the time. Hibbs brought the acetylcholine family of receptors to the lab of Eric Gouaux at Oregon Health & Science University in Portland.
Membrane proteins are notoriously difficult to crystallize. The Gouaux lab’s success with seemingly impossible projects arose from its methodical parallel screening of protein behavior in many conditions with small samples to assay protein behavior before scaling up purification for crystal growth. The Hibbs lab routinely applies these techniques to their cryo-EM studies. He ended up with the first eukaryotic structure in the receptor super family (Nature, 2011).
With a great paper from a top lab, Hibbs interviewed at several universities and received job offers. But by now, Hibbs was married to another scientist, Colleen Noviello, with an infant and toddler born during his postdoctoral fellowship. The cost of childcare ruled out options on either coast.
They surprised themselves by moving to Dallas to start a lab at University of Texas Southwestern Medical Center. Noviello, an infectious disease and immunology specialist, joined as a temporary lab manager and stayed on after they discovered they liked working together. Noviello now leads a branch of the lab on autoimmune disease targeting both acetylcholine and GABA receptors.
In Dallas, Hibbs experienced a supportive environment at an institution with a rich history in structural biology, including the Nobel Laureate Hans Deisenhofer, who determined the first x-ray crystal structure of a membrane protein.
“They treat you really well there, so it was a wonderful place to start my lab,” Hibbs says. “From 2012–2023, we made pretty linear progress and were quickly successful in a lot of different high impact papers, and a lot of that is because the institution was so supportive, with lots of research dollars, and smart, motivated faculty who wanted to see junior faculty succeed.”
Here, also, Hibbs was introduced to the SBGrid resource, where a recent UT Southwestern postdoctoral fellow, Jason Key, was working, and to which already a dozen labs subscribed. “SGBrid was a godsend in making all the computational headaches melt away so that we could focus on the science,” said Hibbs.
They began using cryo-EM when the techniques and technologies enabled high-resolution structures. To gloss over many interesting details, the accomplishments include structures that help explain nicotine addiction (Nature, 2016 & 2018) and how anesthetics and benzodiazepines bind to the GABAA receptor (Nature, 2020).
In a collaboration headed by Noviello, the lab captured the first images of an autoantibody bound to a GABAA receptor, revealing the physical mechanism behind a neurological autoimmune disease (Cell, 2022). The antibody sample came from a physician treating an 8-year-old girl in Germany rendered catatonic by autoimmune encephalitis. (She’s fine now, Hibbs says.)
Next Steps
About this time, Hibbs felt like the lab had answered a lot of its pharmacology questions, with the prospect of incremental returns on their efforts. So they pivoted to answer a different kind of question: “What are the subunit assemblies of these receptors that are found in actual living organisms?” Hibbs explains.
“So why is that important? Drug binding sites are formed at subunit interfaces, mostly. If the order changes, that affects the properties of the receptor, or if you swap one subunit out for another, it changes how much current goes through and the sensitivity to different drugs,” he says.
To answer the new questions, Hibbs returned to the classic biochemical approach of working with tissue, rather than recombinant proteins. That led to the papers on the fetal and adult forms of the muscle receptors that enable calves to stand minutes after birth and to the discovery the heterogeneity of human GABAA receptors and that they are a secondary target of common epilepsy drugs.
And Hibbs has returned to UCSD. In 2023, after feeling squeezed as a family during the pandemic, they moved the lab to San Diego, where Hibbs and Noviello first met as graduate students.
Hibbs now embraces a different set of outdoor muscular activities — surfing, running, mountain biking, and spearfishing. A year after moving, he ran his first 50K trail race. “The struggle here is what to do each day,” he says, “because there are so many good options.”
- By Carol Cruzan Morton

































































































