Two Labs, Many Methods
Michael Sattler
Technical University Munich, Helmholtz Zentrum München
Published April 28, 2015
Two Labs, Many Methods Michael Sattler / Technical University Munich, Helmholtz Zentrum München
In 2011, Michael Sattler took a look at an RNA binding protein that was known, based on earlier X-ray crystallography work, to have a structure with a specific arrangement of two RNA binding domains bound to its RNA ligand. Using nuclear magnetic resonance (NMR) spectroscopy, however, he found at least two different arrangements of the two domains in the protein: one open, one closed, neither resembling that of the crystal structure. “The crystallography missed that there are closed and open states. It’s something you can only find using solution methods,” says Sattler. “This is why we are really committed to using integrated structural biology methods.”
Now, in his dual-role as professor of Biomolecular NMR-Spectroscopy at the Technical University Munich (TUM) and Director of the Institute of Structural Biology at Helmholtz Zentrum München, Sattler has several projects underway — many investigating protein-RNA binding, some using structural biology to guide drug discovery — but one consistent theme is his use of integrated methods. “Everybody now in structural biology is excited about integrated approaches to really get a complete picture of the molecular structures and dynamics,” he says.
Sattler began his career studying chemistry at the University of Frankfurt. As a doctoral student at Frankfurt, he focused on developing NMR spectroscopy methods. He mainly worked with well-characterized, well-behaved model proteins, the best choices for methods development and optimization. “I never made it to look at structures,” he says. “So I decided to get structural biology training as a post-doc.”
In 1995, he started a two-year post-doc at Abbott Laboratories, even though he’d already determined that industry was not where he wanted to end up. He joined the lab of Stephen Fesik, who is now at Vanderbilt but at the time was developing structure-activity relationship (SAR) by NMR, an approach to drug discovery using NMR. While Sattler didn’t work on the method directly, he saw it unfolding around him. It left an impression. “NMR can have an impact on drug development,” he says. “I always kept that in the back of my head.”
What Sattler did focus on at Abbott was the structural biology of apoptosis regulators, such as the Bcl2 protein family. When he created his lab at the European Molecular Biology Laboratory (EMBL) in Heidelberg in 1997, he planned to continue that work. But on arrival, he found himself surrounded by investigators at the forefront of RNA research. “I decided to take the opportunity and start working on RNA binding proteins and protein-RNA interactions,” he says.
For nearly a decade, Sattler made contributions to RNA research, working to tease out how RNA binding proteins uniquely bind to specific RNA and regulate gene expression. He published the structure of splicing factor-1, which is involved in mRNA splicing, and solved a three-dimensional structure of the PAZ domain of the Argonaute protein, which is involved in RNA interference pathways. “I started to realize there are all these interesting RNA binding proteins that are not well understood,” says Sattler, who also expanded his solution-based methods to include small angle x-ray scattering and small angle neutron scattering while at EMBL.
In 2007, Sattler moved into his current, dual role in Munich, with one lab mainly focused on basic science, the other, his Helmholtz lab, focused on translational research and drug discovery. He has access to NMR machines at both labs, small angle scattering at TUM, and a crystallography facility at Helmholtz. The labs are about 10 kilometers apart, but the group meets regularly and members from the two labs work closely together, so the work isn’t completely segregated.
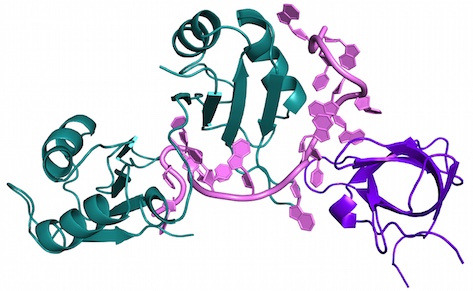
In a 2014 the Sattler group discovered how two proteins bind to an RNA sequence motif in the 3’ untranslated region of an mRNA, creating a protein-RNA sandwich of sorts, to mediate translation silencing of the mRNA. The work serves as a paradigm illustrating how just a few hundred RNA binding proteins may combine together to specifically recognize many thousands of RNA motifs in regulating the expression of our genes. “The binding affinity is increased 1000-fold when both proteins are there,” says Sattler. “The structure shows nicely how you achieve this extreme binding cooperation.”
The work combined X-ray crystallography, NMR and small angle scattering (SAS) in collaboration with researchers in Spain and France. In more current, but not yet published work, Sattler is also fusing NMR and crystallography in a drug discovery project to find small molecule inhibitors that neutralize trypanosoma, parasites that cause sleeping sickness, by disrupting life-critical protein-protein interactions.
-- Elizabeth Dougherty


































































































