Radical reactions
Yvain Nicolet
Institut de Biologie Structurale
Published January 31, 2023
The periodic table may be an icon of chemistry, but a cluster of elements at its center gives biology some essential pop and sparkle. Yvain Nicolet at the Institut de Biologie Structurale (IBS) in Grenoble, France, wants to learn exactly how those transition metals in the middle of the table put more pizzazz in proteins.
About one-third of proteins contain transition metals, such as cobalt, copper, molybdenum, or iron. The Nicolet lab mainly studies metalloproteins that contain iron-sulfur clusters but also scrutinizes enzymes that make these clusters. "Transition metals are often key components of the proteins in terms of function," Nicolet says. "They are only few atoms in a structure and sit in specific sites, but they enable specific activities, notably in enzymes, but not only in enzymes." Metals give proteins some superpowers in the form of new chemical properties and functions not otherwise possible with combinations of the 22 amino acids.
"They enhance the reactivity of the proteins by making many strange active sites that are very interesting," he says. These reactions can be blazingly fast with complex chemistry. And they are technically challenging to study.
The lab investigates enzymes that use metals, as well as proteins that build the metal cofactors and insert them into the enzymes. "We try to trigger the reaction in a crystal and to trap the different intermediates during the reaction to characterize by structural biology," Nicolet says.
Many metalloproteins evolved in a time before oxygen and can only make their razzle-dazzle moves in an anaerobic space. To prevent oxygen from destroying their molecules, experiments in the Nicolet lab take place in a series of six large anaerobic chambers filled with nitrogen.
The researchers reach in through gloves built into the airtight boxes to work with samples and equipment. There, cells are grown and broken up. The cell extracts are cleared and proteins separated by chromatography. Crystallization robots in one glove box screen for optimum conditions that researchers can manually reproduce in another box and freeze in liquid nitrogen. From there, the synchrotron is only 500 meters away.
"We determine 3D structures at moderate to high resolution," Nicolet says. "At first, we aim at determining where the metals are in a given protein, and how they interact with their environment (the protein matrix). We also aim at determining the structure of the metallocofactors or metal binding sites in terms of ligands and geometry.
"Then in a second step, we aim at understanding how these metals confer a specific activity to the protein," he continues. "For instance, in an enzyme, we would get intermediate states with the metal in a different coordinate and/or redox state to better understand what is going on during the reaction."
One milestone of the lab's work arose from successfully trapping an unexpected radical intermediate of NosL, a radical S-adenosyl-L-methionine (SAM) enzyme, in the process of generating a peptide with potent activity against gram-positive bacterial pathogens (Science, 2016).
Crystallography provided the position of the atoms, and for this paper they also used a spectroscopy technique called electron paramagnetic resonance (EPR), which gives the position of unpaired electrons near atoms.
Two months later, the group reported results from a study in which they carried out radical based chemistry catalyzed by the radical SAM enzyme HydE from beginning to end in a crystal (Nature Chemistry, 2016).
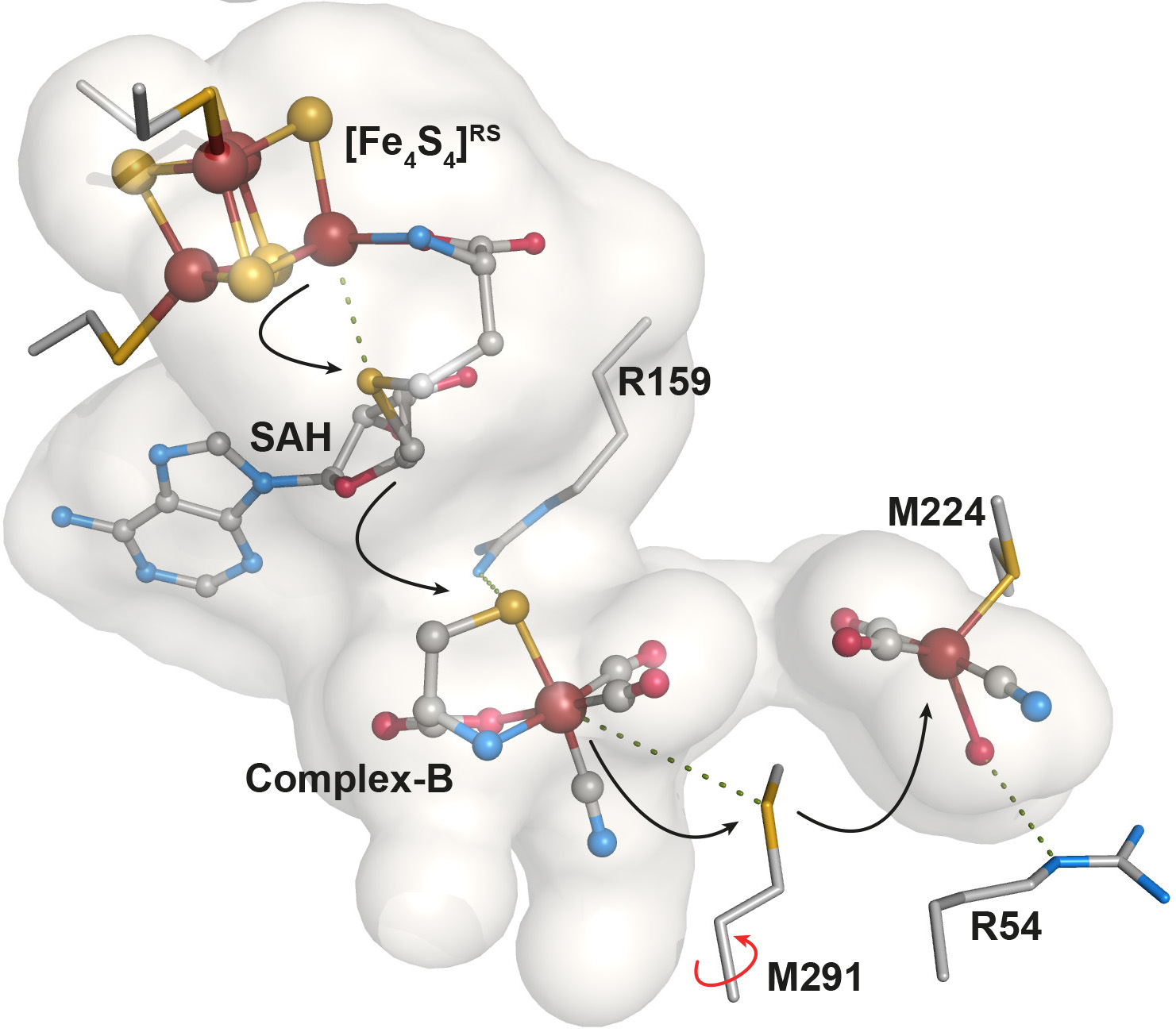
More recently, Nicolet's team characterized intermediates in the assembly of the nitrogenase, a key player in the global nitrogen cycle. The enzymatic complex nitrogenase catalyzes the reduction of nitrogen gas N2 to ammonia NH3, transforming nitrogen and returning it to plants. Specifically, the paper reported the X-ray structure of NifB protein from a nitrogen-fixing bacteria, one of a dozen proteins required to assemble the nitrogenase active site (Journal of the American Chemical Society, May 2020).
"For nitrogenase, there is a dedicated machinery with very, very challenging chemistry involved, and this is what we are attacking over the few next years," he says.
Nicolet was born and raised in Grenoble, France, where he now lives and works. Growing up in the city, he became an accomplished cello player with ambitions of becoming a professional musician. He started college in Grenoble. When a music school in Paris turned down his application, he chose to pursue science at what is now the University of Strasbourg. In Strasbourg, all the science classes emphasized the three-dimensional structures of molecules, whether it was enzymology, DNA replication, or proteins. He completed his undergraduate degree and stayed for graduate training in chemistry, biology, crystallography and nuclear magnetic resonance (NMR).
In a dinner discussion, a friend noted his interest in inorganic chemistry and suggested a structural biology lab in Grenoble working on metalloproteins. He moved back to complete his PhD at what is now the Université Grenoble Alpes. Nicolet worked on the structure of the [FeFe]-hydrogenase (an enzyme that makes or use molecular hydrogen), basic science with a potential application in producing alternative fuel.
From there, he joined the Drennan Lab at the Massachusetts Institute of Technology. In Cambridge, he discovered radical-based chemistry working on this newfound Radical SAM Proteins superfamily, now known for their ability to synthesize many vitamins, cofactors, or antibiotics.
He moved back to Grenoble for a one-year postdoctoral fellowship with the European Synchrotron Radiation Facility. In 2004, he joined IBS as a scientist and became a group leader there in 2016.
The Nicolet lab often dives into the molecular mechanisms of phenomenon discovered by other groups. "In my lab, we are not at the beginning of the discovery, and we are probably not at the end of the process," he says. "We focus on the molecular mechanisms inside the enzymes."
In a new challenge, the lab has set up a system to use cryo-electron microscopy to examine the assembly dynamics and reactions at room temperature without oxygen. Nicolet is interested in the basic science of radical chemistry, but he also hopes to be able to harness a radical enzyme and modify its catalytic activity to produce a molecule through directed evolution, such as a better antibiotic.
Nicolet also enjoys taking a bigger picture view of the nitrogen cycle, thanks to his garden. He came late to the activity and confesses that he is not good at growing vegetables. "You can observe the nature or the interaction between the different organisms that live together from bacteria to insects and plants," he says. "For me, it's the best way to contemplate what Darwin described as natural selection. And evolution. I'm not very good at producing vegetables, but I really enjoy seeing how all these organisms compete together or help each other as well.
-Carol Cruzan Morton































