Death Metal
Steven Damo
Fisk University
Published April 28, 2024
For many people, the phrase “nutritional immunity” may evoke special foods or vitamin supplements believed to boost immunity, such as chicken soup, vitamin C, or tea with honey.
But for Steven Damo at Fisk University in Nashville, it refers to a tactic the immune system employs to starve bacteria of vital trace metals they need to survive.
Damo’s research focuses on battles waged between the immune system and pathogenic bacteria. “When we get sick, how does our immune system fight off infection?” Damo asks. “And then on the flip side, how do bacterial pathogens utilize different virulence factors to circumvent the host immune response and continue their pathogenesis?”
Specifically, he’s interested in how the host and pathogen wield essential micronutrients, such as zinc and manganese. All living organisms need these elements, but microbial pathogens must acquire them from their host.
“In terms of basic science, metals are important cofactors, and they're signaling ions, and maybe a third of the proteome is predicted to be a metallic protein,” he says. “So not having access to metals would clearly stunt bacterial growth. But on the flip side, too much metal is actually toxic to cellular environments, and our immune system actually also leverages this fact as well."
In the long-running struggle between immune defenses and bacteria countermoves, trace metals are both the prize and the weapon. An example of nutritional immunity involves the most abundant protein, calprotectin, deployed by the most abundant immune cells, neutrophils.
The protein concentrates at sites of infection, where it sponges up available manganese that would otherwise nourish the bacteria. As a postdoctoral fellow, Damo identified the molecular basis of manganese sequestration in a calprotectin structure he determined.
Bacteria Defuse Metal Overload
More recently, Damo and his collaborators investigated how some bacteria evade another immune tactic, metal intoxication. To ward off potential infections, innate immune cells called macrophages engulf bacteria and flood them with toxic levels of zinc.
In this case, the researchers wanted to know more about Group B Streptococcus infection in pregnancy. Group B Strep is a common gastrointestinal microbe, but somehow it can get past immune defenses into the placenta and fetal membranes in an infection associated with preterm birth, neonatal sepsis and stillbirth.
They discovered that Group B Strep not only survive macrophages, it exploits them. In an encounter with the immune cell, the bacteria turn on a protein called cadD, which drains the poisonous zinc. Then, the bacteria ride inside macrophage like a Trojan horse to infiltrate other tissues, including the vulnerable fetus. (Nature Communications, 2022)
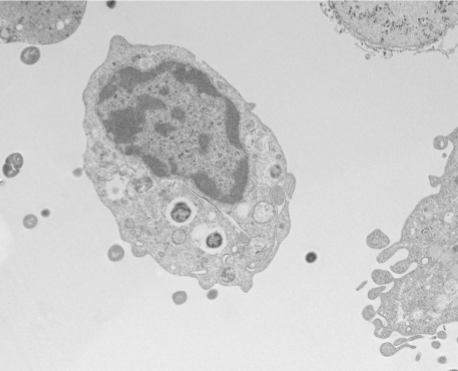
Damo’s lab is following up with structural studies of the protein and the zinc efflux mechanism. The research team included Jennifer Gaddy at Vanderbilt University, David Aronoff at Indiana University, Shannon Manning at Michigan State University, and others.
“In my lab, we focus on trying to explain molecular mechanisms of interesting proteins, but we're highly collaborative,” Damo says. “We work with microbiologists, physicians, data scientists, trying to get as many perspectives as possible to look at the host-pathogen interface.”
From Chemistry to Biology
Math and science were some of Damo’s favorite subjects as far back as elementary school, partly because they came easy to him. Even in high school, if he paid attention in class, he didn’t have to study—until AP chemistry.
“It was the first time I didn’t get straight A’s on my report card,” he says. But he had already paid to take the upcoming AP exam. So he went to the bookstore and bought an AP test prep workbook.
“I basically cut class for two weeks,” he says. “I did every single problem in that workbook. And I read the textbook for the first time. I essentially taught myself the AP chemistry curriculum, and I wound up scoring remarkably well on the exam.”
The experience was transformative in more ways than one. First, he realized he could learn things on his own, an epiphany that still resonates with him as a professor. “The teacher is a guide, and they can help you,” he says, “but it's really your independent ability to do the reading, do the research, and put the work in—you can teach yourself these things.”
Also, he learned he liked chemistry. A lot. As a freshman at New York University (NYU), he chose chemistry as his major and ensconced himself in a research laboratory for the next three years.
Growing up in Queens in New York City, Damo didn’t know any scientists, and it never occurred to him as a career option. But he thrived under the tutelage of the engaging and supportive science faculty he encountered at NYU. And, he quickly saw, lab bench work was unlike the sorts of jobs he had worked before—fast food, warehouse, pasta factory—where he did what his boss told him to do.
“I had no idea what it meant to be a scientist,” Damo says, “but as early as an undergrad, you're given a lot of respect and power. Because your project is your project, and no one gives you a project they know the answer to. When you're in the lab, you have your ideas, and you're free to pursue them—within reason of course—but you can really make a significant contribution in terms of the science, and that’s very different from other professions.”
In his senior year, he switched labs and began working with Marc Walters, an inorganic chemist who modeled metal centers in proteins and characterized them using vibrational spectroscopy. Damo was dazzled by the idea of shining a laser on a sample and using mathematics to make sense of the data. In Walters’ lab, he was tasked with making a molecule needed in those experiments. The molecular recipe was buried in German journals, written in a dialect so archaic that even a native German postdoctoral fellow in the lab puzzled over the syntax. Together, they extracted enough of the methodology to develop a protocol.
The molecule also turned out to be Damo’s gateway into structural biology when he ran other experiments to create novel metal complexes. He used X-ray crystallography, nuclear magnetic resonance (NMR), and mass spectroscopy to explain their physical properties.
For graduate school, Damo turned to physical chemistry at University of California, Berkeley. He landed in the lab of David Wemmer, who had developed NMR methods to study protein structure. Damo’s interest evolved from the technology to the application—in this case, amyloid proteins, such as those implicated in Alzheimer’s and prion diseases.
After grad school, Damo aspired to work on a biological system important in health and medicine. He completed two postdoctoral fellowships, one with Hao Wu, then at Weill Cornell Medical College, New York, and now at Harvard Medical School, Boston, and then with Walter Chazin at Vanderbilt University in Nashville.
The move from New York City to the San Francisco Bay Area had been a big culture shock for Damo. When he was writing his dissertation, he started to study latin dance to connect with the music and culture he missed. During his New York postdoc, he joined a dance company and participated in Off Broadway productions and cultural events. A highlight was performing at the United Nations. When he first moved Nashville, he connected with friends of mutual friends and started a dance company, where he performed and taught classes. When he joined the Fisk and Vanderbilt faculties, he retired as a latin dancer, but he remains on the board of the Global Education Center, a multicultural organization in Nashville dedicated to using the arts to highlight the commonalities among people.
As a postdoc in the Chazin lab, he determined the crystal structure of calprotectin bound to manganese. (Proceedings of the National Academy of Sciences, 2013)
“In most manganese metalloproteins, the manganese is buried somewhere in the core of the protein,” Damo says. “But calprotectin functions very different. Its job is to actually reach out and grab free manganese and prevent other proteins from getting to it. This is an incredibly potent anti-microbial strategy. If you basically tried to grow any bacteria—and we had half a dozen different human pathogens, and you just put recombinantly made purified calprotectin in the test tube with the bacteria, the bacteria won't grow, because they can't get the metal from the media.”
Looking forward, Damo wants to study these metal protein mechanisms in their natural habitats, such as cells, using cryo-electron microscopy (cryo-EM).
Training the Next Generation
Damo has an adjunct appointment at Vanderbilt University, which gives him access to core research facilities, including cryo-EM. Fisk is primarily an undergraduate institution and a historically black university. The graduate students in his lab are mostly master’s students in a master’s-to-PhD bridge program.
When Damo was on the job market, the combination of research and mentorship Fisk offered was irresistible. Damo’s degrees and training took place at large graduate institutions—NYU, Berkeley, Cornell, and Vanderbilt. “As someone who grew up not being a scientist, I was very cognizant of my integration and acceptance into this community,” Damo says. “As I went through my training, I noticed that I was always one of the few. So if I could be at an institution where everyone looks like me, and some would have to face similar experiences as me, I could leverage my personal experiences to teach them to become the scientist that they want to be.”
Damo describes the graduate programs as a national model for diversifying STEM. (American Journal of Physics, 2011) “It turns out people from historically excluded backgrounds will frequently get a master's en route to the PhD, and they'll often do it at different institutions,” he says. “This isn’t the standard path, right? Usually, you go straight to a PhD program. A master’s is like you’re in a PhD program and you want to leave, so okay, here’s your master’s.
After that observation was made about 20 years ago, Fisk and Vanderbilt formalized it into a graduate training problem in which students first earn a master’s and then go on to a PhD at Vanderbilt or another institution, such as the University of California, Los Angeles or Johns Hopkins University in the case of students from the Damo lab.
Damo estimates about half of Fisk students are first generation in their families to attend college. “These are students who have talent and interest in pursuing scientific careers but often aren't encouraged or able to navigate the path for whatever reason,” he says. “We have to remember that science does not exist in a vacuum. Science is done by scientists who are people. Once you introduce the humanity of it, all this stuff that we say we ignore, we can't—including bad things like biases, systemic racism, and also the good things, the collaborations, the friendships, the privilege—all that now becomes part of the science as well."
“When we think about our scientific workforce, there are people who will have the talent and have the desire to become scientists,” he says. “But they need a way to find the path to get them there. That's just as much a part of my job as making the discoveries. In fact, from my perspective, my research program isn't for the sake of the research program, it's the vehicle I use to provide these kinds of opportunities for those students.”
-Carol Cruzan Morton






























