Jumping Genes
Orsolya Barabas
European Molecular Biology Laboratory, Heidelberg
Published June 27, 2018
Thickly forested slopes define the environs around Heidelberg, Germany, the headquarters of the European Molecular Biology Laboratory. In her hillside EMBL laboratory, Orsolya Barabas probes the small pieces of moveable DNA that define the landscape of modern genomes.
Surprisingly few people have heard of transposons. These “jumping genes” have been flitting around genomes for millions of years, changing locations or introducing new copies of themselves all over the place in plants and animals. They escaped notice until the 1940s, when Barbara McClintock observed them in maize, where they dominate the DNA, eventually earning her a Nobel Prize in 1983.
At the last best guess, about half of the human genome is composed of mostly dormant remnants of transposons. Bacteria, on the other hand, rely on highly active transposons to share genes to help them adapt and survive, such as those that confer antibiotic resistance.
“Transposons are very simple DNA vehicles that can help integrate genetic cargo into the genome,” says Barabas, who works both sides of the potential medical applications, starting from the atomic details. Her group studies transposons used in genetic engineering, seeking to make their gene delivery more efficient. On the flip side, she also scrutinizes transposons that spread antibiotic resistance genes among bacteria, hoping to limit that activity.
The scientific world has been buzzing about CRISPR/Cas9 for targeting and editing a specific location on DNA, but transposons have the edge when it comes to the step of inserting a gene, Barabas says. Transposons also have some advantages over viral vectors, a more established way to insert genes efficiently.
“Transposons are the first line of non-viral gene delivery tools,” she says. “Their applicability is greatest for ex vivo cell engineering.” The early use of transposons has mostly been in personalized immunotherapy clinical trials, in which a patient’s immune cells are withdrawn, genetically altered to recognize and kill cancer cells, and then injected back into the patient.
Barabas and her colleagues have found a way to improve the first and most popular such tool. Called Sleeping Beauty, the transposon was synthesized about two decades ago by Hungarian researchers. The awakened transposon was reconstructed from ancient DNA sequences estimated to be more than 10 million years old, which had been fished out of the genomes of trout and salmon. In their lab in Germany, these same researchers have since modified Sleeping Beauty to be 100 times more efficient, a version called SB100X.
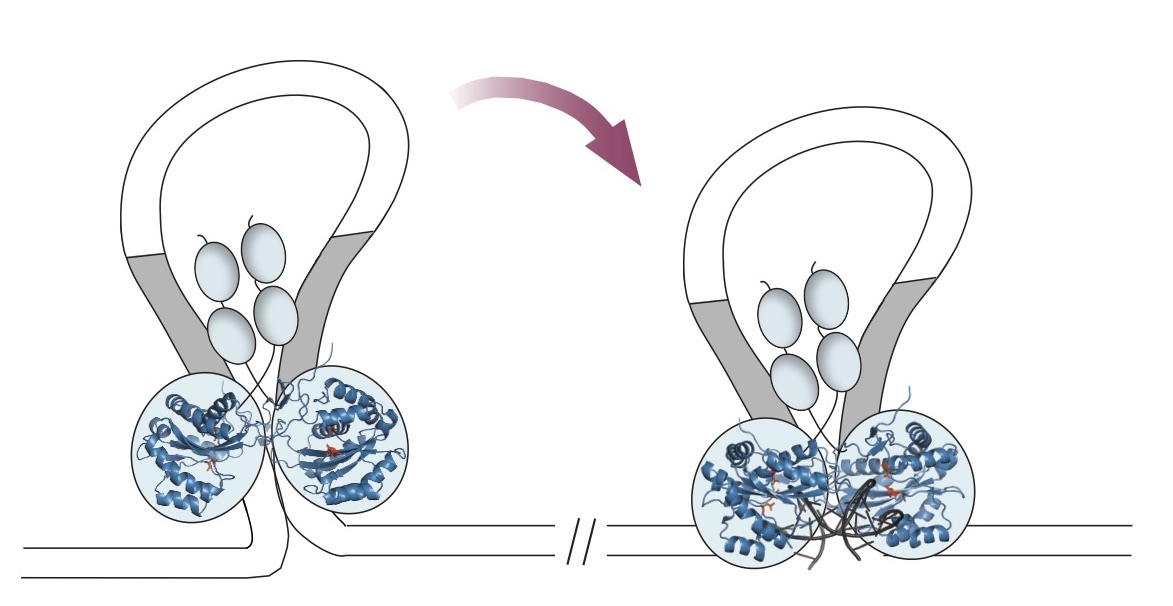
In a 2016 paper in Nature Communications, Barabas and her co-authors reported the first crystal structure of the key piece, the SB100X transposase enzyme. Transposases are the workhorses of transposons, clamping, cleaving, and rejoining the DNA.
Using the new structure, the team tweaked some amino acids on the surface of the enzyme to make versions that could bind even better to the DNA where genetic integration occurs.
“They were the first rationally designed hyperactive transposon variants,” she says. The new design has a 30 percent efficiency boost. These variants are not yet in cancer trials, but for patients waiting for cancer treatment, Barabas estimates they may shave as much as a week off a month-long wait for SB100X to supercharge their immune cells.
“As well adapted parasites, transposase enzymes are not naturally optimized to work efficiently,” she adds. “This leaves a lot of room for optimizing their activities for human use or genome manufacturing at will.”
One limitation of transposons for genetic engineering is that they insert cargo in many different places on the DNA. In contrast, the CRISPR/Cas9 system can target a specific DNA site, but then it relies on the host’s DNA repair mechanism to insert a gene, which isn’t good enough for a medical setting, she says.
“Ideally, it would be nice to combine these things: Site specificity and integration,” Barabas says. Until that vision becomes reality, her lab continues to develop more hyperactive variants and seek ways to target them better.
A different project in the lab has the opposite goal: To derail transposon activity—at least those that spread antibiotic resistance among bacteria. The numbers of microbes resistant to multiple antibiotics has been increasing globally, causing concern among scientists and doctors about the threat to public health.
“The fact is that resistance genes already exist for all antibiotics we use,” she says. “There is not a single antibiotic for which a resistance mechanism does not exist.”
Transposons largely drive the spread of antibiotic resistant genes among bacteria, even within the resident microbes in our gut. Stress—such as antibiotic treatment—can trigger bacterial transposons to amplify.
The Barabas lab started with conjugative transposons, which move between the single-cell microbes during the process scientists jokingly refer to as bacterial sex. Normally, two single-celled microbes meet and form a membrane tube to swap DNA and proteins, including transposons carrying genes that confer drug-resistance.
They solved the first structure of a transposon of this class. A 2018 Cell paper reported the transposon DNA in conjunction with the protein machinery that plucks out resistance genes. The structure shows how the enzyme can peel open the DNA double helix in two parts. A single stranded segment forms a bubble that can touch down almost anywhere else without needing to match a specific sequence.

Based on the structure, Barabas and her colleagues predicted two types of molecular inhibitors. One peptide blocks the transposase protein from moving to its activated conformation. The second is a small DNA and binds to the open site within the transposon, blocking the DNA strand replacement needed for resistance transfer.
She envisions potential inhibitors may be used in combination with a course of antibiotics, to prevent resistance spreading.
Barabas grew up in Hungary, the daughter of two chemical engineers. She remembers being fascinated by how things in everyday life could be explained by chemistry and other phenomena people can’t normally see. When it came time to train for a career, Barabas decided to direct her imagination and creativity toward chemistry and basic research. Her father was concerned, reasoning that the country could not support many basic researchers.At Eötvös Loránd University in Budapest, Barabas studied structural chemistry. For her masters, she crystalized small molecules used in drugs and pills, learning how their structure influences their actions and effects. Then she moved on to enzymes, looking at their effect on nucleic acid metabolism and maintenance. When she finished her PhD in 2005, she was riveted by how proteins juggle with DNA.

She crossed the Atlantic Ocean for a postdoctoral position at the National Institutes of Health in Bethesda, Maryland, USA and was introduced to transposons in the lab of Frederick Dyda. “I had no idea what transposons were, but they sounded fascinating—dedicated elements in the genome that fancy to hop around, shaping us into who we are,” Barabas says. She became a group leader at EMBL in 2009. Over time, Barabas has become increasingly intent on connecting basic structural knowledge to applications.
“It’s important that we really use the structure to understand its function, and also develop it into something useful for society,” she says. “For me, the applicability of the acquired knowledge is critically important. Taxpayers pay us to have the most thrilling job, and I need to pay them back.” To help push past the basic discoveries, the Barabas lab does biochemistry, biophysics, structures, cell biology and other methods.
Collaborations abound. To explore how the antibiotic slaughter of gut bacteria affects their susceptibility to antibiotic resistance genes, she has teamed up with computational geneticists and a mouse biology lab, as well as with a clinician for human stool samples. This will allow investigating how transposons spread in microbial communities in the gut and what triggers induce the movements. More structures are on the menu to compare mechanisms among the many different transposons.
-Carol Cruzan Morton
Other tales
-
![]()
The Stories Antibodies Tell
Jean-Philippe Julien
Published 27 January 2026
![]()
Reshaping Membranes
Melanie Ohi
Published 23 November 2025
-
![]()
Probing Microbes
Gira Bhabha
Published 30 September 2025
![]()
Drawn to the Light
Emina Stojković
Published 30 July 2025
-
![]()
The Final Phase
George Phillips
Published 31 May 2025
![]()
Mind and Muscle
Ryan Hibbs
Published 28 March 2025
-
![]()
The Shapes of Energy
Luke Chao
Published 12 December 2024
![]()
Predicting Proteins
Jens Meiler
Published 25 November 2024
-
![]()
Death Metal
Steven Damo
Published 28 April 2024
![]()
Context Matters
Bing Chen
Published 30 January 2024
-
![]()
The Crystal Whisperer
Sarah Bowman
Published 29 November 2023
![]()
Data in Motion
Nozomi Ando
Published 29 September 2023
-
![]()
The Monstrous Maw
André Hoelz
Published 28 June 2023
![]()
Second Takes
Andrea Thorn
Published 28 February 2023
-
![]()
Radical reactions
Yvain Nicolet
Published 31 January 2023
![]()
Floppy Physics
Eva Nogales
Published 30 November 2022
-
![]()
Structure of Equity
Jamaine Davis
Published 28 September 2022
![]()
Life and Death of a Cell
Evris Gavathiotis
Published 28 July 2022
-
![]()
Follow the glow
Kurt Krause
Published 29 April 2022
![]()
Resolution solutions
Willy Wriggers
Published 25 February 2022
-
![]()
Of enzymes and membranes
Ming Zhou
Published 28 October 2021
![]()
Step-by-step
Gabrielle Rudenko
Published 26 September 2021
-
![]()
Moving muscle
Montserrat Samso
Published 26 July 2021
![]()
Particle catcher
Stefan Raunser
Published 28 June 2021
-
![]()
Designer drugs
Ho Leung Ng
Published 25 February 2021
![]()
Right place, right time
Ernesto Fuentes
Published 29 January 2021
-
![]()
Shape-shifting secrets of membranes
James Hurley
Published 27 November 2020
![]()
Enzymatic action
Cynthia Wolberger
Published 28 September 2020
-
![]()
Rules of motion
Priyamvada Acharya
Published 31 July 2020
![]()
Cosmic Squared
Michael Cianfrocco
Published 27 June 2020
-
![]()
Kaps are Cool
Yuh Min Chook
Published 28 April 2020
![]()
Spiraling into focus
Carsten Sachse
Published 29 March 2020
-
![]()
Seeing cilia
Alan Brown
Published 27 February 2020
![]()
For the Love of EM
Guy Schoehn
Published 27 January 2020
-
![]()
Protein Puddles
Michael Rosen
Published 16 December 2019
![]()
Changing channels
Daniel Minor Jr.
Published 27 September 2019
-
![]()
Listening Tips
Marcos Sotomayor
Published 30 July 2019
![]()
Beyond Cool
Published 31 May 2019
-
![]()
Hao Wu
A Higher Order
Published 30 May 2019
![]()
Aye Aye Captain
Alexandre Bonvin
Published 29 April 2019
-
![]()
The PARP Family Family
John Pascal
Published 28 February 2019
![]()
Frame by frame
Nikolaus Grigorieff
Published 28 January 2019
-
![]()
Predicting Success
Bil Clemons
Published 18 December 2018
![]()
Curiouser and Curiouser
Ramaswamy Subramanian
Published 27 November 2018
-
![]()
Rely on This
Sjors Scheres
Published 26 October 2018
![]()
Proteins out of bounds
Gerhard Wagner
Published 27 September 2018
-
![]()
Hiding in plain sight
Gaya Amarasinghe
Published 27 July 2018
![]()
Jumping Genes
Orsolya Barabas
Published 27 June 2018
-
![]()
Data Whisperer
Karolin Luger
Published 30 May 2018
![]()
Flipping the Switch
Jacqueline Cherfils
Published 27 April 2018
-
![]()
Tooling Around
Andrew Kruse
Published 29 March 2018
![]()
Comings and Goings
Tom Rapoport, Ph.D.
Published 23 February 2018
-
![]()
Transcriptional Rhythm
Seth Darst
Published 27 January 2018
![]()
The Language of Gene Regulation
Daniel Panne
Published 21 November 2017
-
![]()
Not Your Average Protein
James Fraser
Published 23 October 2017
![]()
Message Received
Sebastien Granier
Published 24 August 2017
-
![]()
Resistance is Futile
Celia Schiffer
Published 28 July 2017
![]()
Twist of Fate
Leemor Joshua-Tor
Published 28 June 2017
-
![]()
Drug Designer
John Buolamwini
Published 30 May 2017
![]()
Mathematically Minded
James Holton
Published 28 April 2017
-
![]()
Garbage Out
Kay Diederichs
Published 30 March 2017
![]()
Fixer Upper
Brandt Eichman
Published 27 February 2017
-
![]()
Mobilizers
Phoebe Rice
Published 31 January 2017
![]()
Escape Artist
Katya Heldwein
Published 19 December 2016
-
![]()
Nature’s Confectioner
Jochen Zimmer
Published 29 November 2016
![]()
State of Fusion
Jason McLellan
Published 27 October 2016
-
![]()
Here Be Dragons
Brian Fox
Published 28 September 2016
![]()
SBGrid Assumes Ownership of PyMOLWiki
Published 15 September 2016
-
![]()
Pharm Team
Oleg Tsodikov
Published 24 August 2016
![]()
Spiro-Gyra
Alejandro Buschiazzo
Published 27 July 2016
-
![]()
Turning the DIALS
Nicholas Sauter
Published 29 June 2016
![]()
Pipeline Dreams
Bridget Carragher and Clint Potter
Published 26 April 2016
-
![]()
U-Store-It
The SBGrid Data Bank provides an affordable and sustainable way to preserve and share structural biology data
Published 28 March 2016
![]()
Big Questions, Big Answers
Jennifer Doudna
Published 22 February 2016
-
![]()
Not a Structural Biologist
Enrico Di Cera
Published 17 December 2015
![]()
Divide and Conquer
Kevin Corbett
Published 19 November 2015
-
![]()
Computing Cellular Clockworks
Klaus Schulten
Published 23 October 2015
![]()
Trans-Plant
Gang Dong
Published 26 September 2015
-
![]()
Keep on Moving
James Berger
Published 23 August 2015
![]()
Totally Tubular
Antonina Roll-Mecak
Published 27 July 2015
-
![]()
From Disorder, Function
Julie Forman-Kay
Published 29 June 2015
![]()
Into Alignment
Geoff Barton
Published 27 May 2015
-
![]()
Two Labs, Many Methods
Michael Sattler
Published 28 April 2015
![]()
Picture This
Georgios Skiniotis
Published 20 March 2015
-
![]()
Intron Intrigue
Navtej Toor
Published 20 February 2015
![]()
Cut and Paste
Martin Jinek
Published 28 January 2015
-
![]()
Basics and Beyond
Qing Fan
Published 18 December 2014
![]()
Bloodletting and Other Studies
Pedro José Barbosa Pereira
Published 25 November 2014
-
![]()
Wire Models, Wired
A brief history of UCSF Chimera
Published 29 October 2014
![]()
In Search of…New Drugs
Doug Daniels
Published 30 September 2014
-
![]()
An Affinity for Affinity…and Corals
John C. Williams
Published 29 August 2014
![]()
Pete Meyer, Ph.D.
Research Computing Specialist
Published 22 August 2014
-
![]()
Justin O'Connor
Sr. System Administrator
Published 20 August 2014
![]()
Carol Herre
Software Release Engineer
Published 15 August 2014
-
![]()
Elizabeth Dougherty
Science Writer
Published 13 August 2014
![]()
Andrew Morin, Ph.D.
Policy Research Fellow
Published 11 August 2014
-
![]()
Jason Key, Ph.D.
Associate Director of Technology and Innovation
Published 8 August 2014
![]()
Piotr Sliz, Ph.D.
Principal Investigator, SBGrid
Published 1 August 2014
-
![]()
New Kid on the Block
James Chen
Published 29 July 2014
![]()
Membrane Master
Tamir Gonen
Published 30 June 2014
-
![]()
The Natural Bridge
Piotr Sliz
Published 13 June 2014
![]()
Surprise, Surprise
Catherine Drennan
Published 26 April 2014
-
![]()
Gone Viral
Olve Peersen
Published 20 March 2014
![]()
All Who Wander Are Not Lost
Frank Delaglio
Published 24 February 2014
-
![]()
The Raw and the Cooked
Graeme Winter
Published 24 January 2014
![]()
Vacc-elerator
Peter Kwong
Published 17 December 2013
-
![]()
Structural Storyteller
Karin Reinisch
Published 15 November 2013
![]()
The Fixer
Jane Richardson
Published 28 October 2013
-
![]()
Inside the Box
Mishtu Dey
Published 17 September 2013
![]()
Sensing a Change
Brian Crane
Published 16 August 2013
-
![]()
Towards Personalized Oncology
Mark Lemmon
Published 16 July 2013
![]()
Brush with Fame
Yizhi Jane Tao
Published 14 June 2013
-
![]()
Toxic Avenger
Borden Lacy
Published 21 May 2013
![]()
Pushing the Boundaries
Stephen Harrison
Published 22 April 2013
-
![]()
Strength in Numbers
Joseph Ho
Published 18 March 2013
![]()
One Lab, Many Methods
Wesley Sundquist
Published 12 February 2013
-
![]()
Unplanned Pioneer
Tim Stevens
Published 15 January 2013
![]()
From Actin to Action
Emil Pai
Published 11 January 2013
-
![]()
Unstructured
A Brief History of CCP4
Published 12 December 2012
![]()
Stop, Collaborate and Listen
Eleanor Dodson
Published 5 November 2012
-
![]()
X-PLORer
Axel Brunger
Published 1 October 2012
![]()
Share the Wealth
Zbyszek Otwinowski
Published 22 August 2012
-
![]()
Unraveling RNA
Anna Pyle
Published 18 July 2012
![]()
Sharper Image
Pawel Penczek and SPARX
Published 4 June 2012
-
![]()
Creative Copy Cat
Pamela Bjorkman
Published 25 April 2012
![]()
Charm and Diplomacy
Gerard Kleywegt
Published 7 March 2012
-
![]()
From Curiosity to Cure
Marc Kvansakul
Published 13 December 2011
![]()
The Lure of the Sandbox
Paul Emsley and Coot
Published 15 October 2011
-
![]()
Springsteen, Tolkien, Protein
Alwyn Jones and Frodo
Published 17 June 2011
![]()
Structures Solved Simply
Paul Adams and Tom Terwilliger on Phenix
Published 2 June 2011
-
![]()
Playing the Odds
Randy Read and Phaser
Published 19 May 2011
![]()
Escape from the Darkroom
Wolfgang Kabsch and XDS
Published 19 May 2011
-
![]()
Better, Faster, Stronger, More
Victor Lamzin and ARP/wARP
Published 17 May 2011
![]()
Crystallography for Kids
Lynne Howell
Published 17 May 2011




































































































































