For the Love of EM
Guy Schoehn
Institut de Biologie Structurale, Grenoble, France
Published January 27, 2020
One day soon, the process of collecting data from cryo-electron microscopes may be as automated as it is on synchrotron beamlines. Until then, Guy Schoehn and his team will be busy training and collaborating with other researchers seeking technical EM expertise and insights.
Schoehn manages the EM platform at Institut de Biologie Structurale (IBS) in Grenoble, France. In December 2018, the institute upgraded its EM platform with a powerful new €2-million transmission electron microscope to support growing demand for higher resolution cryo-EM.
The new equipment marks a milestone for Schoehn and the institute. “We are quite lucky to have this here,” he says. “France is under-equipped, so there is a lot of pressure on the very good microscopes we have.”
Schoehn understands the popularity of the new in-house EM platform. As a graduate student in the late 1990s, he was attracted to electron microscopy, even though, at 20-40 angstroms resolution, those early molecular images were little more than blurred blobs.
“With EM, you could actually get images,” Schoehn says. In contrast, “if we’re talking about X-ray, you see diffraction patterns—basically some spots. In NMR, you see a spectrum. But you never directly see the sample. In EM, you can directly see what you’re working on. That’s quite magic.”
Since then, the magic has only grown more powerful. His career trajectory has paralleled the “resolution revolution” in cryo-EM, as advances in technology and techniques have enabled scientists to begin to explore molecular structures in atomic detail.
Schoehn grew up near Strasbourg in a small village. His father worked deep in the now depleted potash mines, a major source of potassium used worldwide in fertilizer. As a child, Schoehn remembers always being curious, especially about biology.
In the mid-1990s, his undergraduate studies in biochemistry at Joseph Fourier University first brought him to Grenoble. He stayed in town for a PhD in physics at IBS with Elizabeth Hewat, the only place in France then combining structural virology and cryo-EM.
He left France briefly, for a 15-month postdoctoral fellowship in the London lab of Helen Saibil. He returned to the Grenoble EMBL Oustation with Rob Ruigrok for further postdoctoral studies and eventually settled in as a permanent staff scientist. He met his future wife at IBS during his PhD studies, and she also has a permanent position on the EM team as a research engineer. In 2014, Schoehn became the EM group leader at the IBS, overseeing three teams and the technical platform, with a goal of facilitating access.
For the new EM, a lot of work went on behind the scenes to convince decision makers of its usefulness, to connect with other department heads to find the money, to prepare a specialized room, and to build out the computational capacity with the help of the IT team. After all, each three-day experiment generates 2 terabytes of data.
Once the microscope arrived, he performed testing to make sure everything worked correctly. They undertook the training they needed to train others. Nearly all the groups in the institute wanted access, plus a few more, creating a two-month waiting list.
Beyond training their peers, Schoehn’s group collaborates with more than a dozen other IBS structural biology labs, providing scientific input as well as EM expertise and image analysis to answer a biological question with a sample provided by the collaborator. The lab also has in-house projects on viruses.
“My main interest was and is always viruses,” he says.
One collaboration is systematically unpacking how the measles virus replicates. Despite effective vaccines, measles is a highly contagious disease that kills more than 100,000 people a year. In a paper in Science, the IBS labs of Schoehn, Irina Gutsche and Ruigrok reported a high-resolution structure of the measles capsid, the protein package containing the virus genome that invades and infects the host cells.
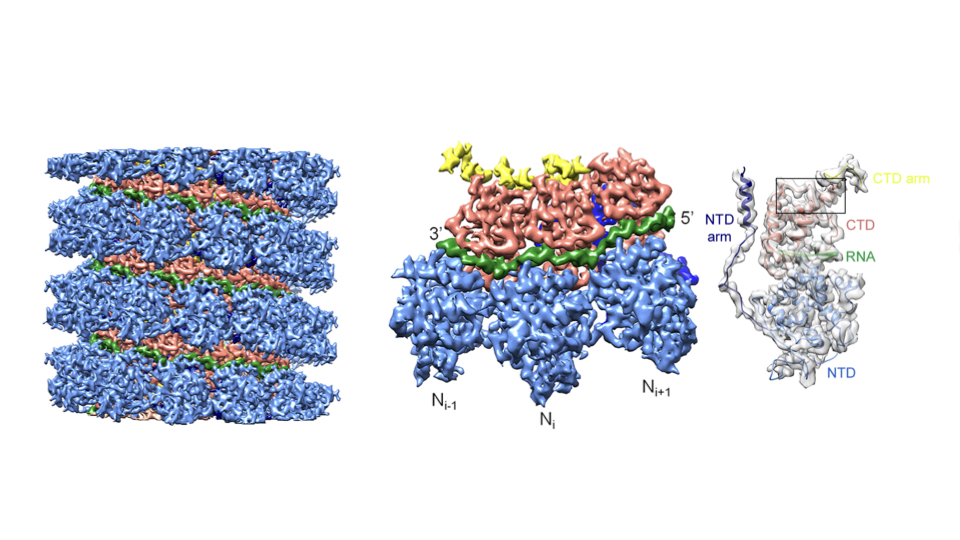
The nearly atomic detail showed how several capsid nucleoproteins assemble in a helical shell around the viral RNA, and how the capsid protein and viral RNA interact. At 4.3 angstrom, the structure stands out for Schoehn as the first atomic-scale EM solution from his group. The findings may inform drug design. The project continues, also in collaboration with Martin Blackledge’s group at IBS.
More than two dozen other papers reflect the lab’s long-standing interest in adenoviruses—viruses that cause common respiratory infections but can be altered to become therapeutic vectors. For example, an adenovirus vector delivered the first effective gene therapy, and the tool is showing promise as a way to deliver oncolytic viruses that preferentially target cancer cells.
In 2005, Schoehn reported the EM-solved structure of an entire adenovirus capsid. Fast forward to 2019, when he and Pascal Fender, a team leader in his group, reported the atomic structure of a complex between the adenovirus and the cell receptor. The findings, reported in Nature Communications, explain how the virus recognizes and interacts with the cell. The structure (about 100 kDa) was one of the smallest complexes solved with cryo-EM at the time.
Another project centers on HIV. When HIV infects a human cell, the virus enters, replicates, and leaves the cell. The exit is called “budding,” and is governed by the virus. In collaboration with the IBS lab of Winfried Weissenhorn, Schoehn’s lab is looking at several proteins involved in the budding, using a similar virus to probe the process.
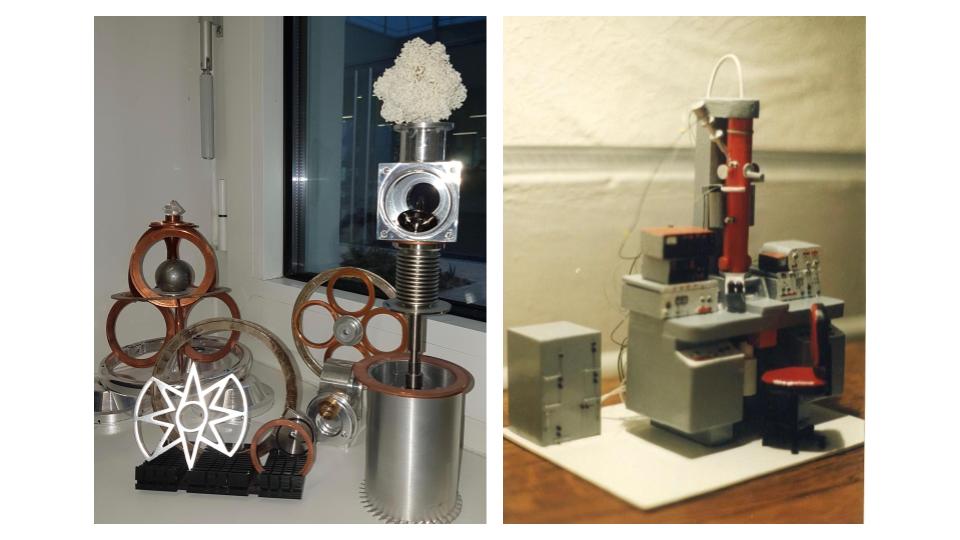
On his own time, Schoehn occasionally relaxes by constructing detailed structures of his own design. He once crafted a miniature balsa wood model of his first EM for the technician who helped him during his PhD in 1994. He now creates more whimsical objects, including a clock made from music compact discs (CDs) and Kinder figurines (little children’s toys packed in German chocolate eggs sold as Kinder Surprise) or a tiered moving sculpture made from beer cans, an old electrical motor, spare pieces of old electron microscopes and “a whole bunch of other stuff from flea markets,” Schoehn says.
-Carol Cruzan Morton
Other tales
-
![]()
The Stories Antibodies Tell
Jean-Philippe Julien
Published 27 January 2026
![]()
Reshaping Membranes
Melanie Ohi
Published 23 November 2025
-
![]()
Probing Microbes
Gira Bhabha
Published 30 September 2025
![]()
Drawn to the Light
Emina Stojković
Published 30 July 2025
-
![]()
The Final Phase
George Phillips
Published 31 May 2025
![]()
Mind and Muscle
Ryan Hibbs
Published 28 March 2025
-
![]()
The Shapes of Energy
Luke Chao
Published 12 December 2024
![]()
Predicting Proteins
Jens Meiler
Published 25 November 2024
-
![]()
Death Metal
Steven Damo
Published 28 April 2024
![]()
Context Matters
Bing Chen
Published 30 January 2024
-
![]()
The Crystal Whisperer
Sarah Bowman
Published 29 November 2023
![]()
Data in Motion
Nozomi Ando
Published 29 September 2023
-
![]()
The Monstrous Maw
André Hoelz
Published 28 June 2023
![]()
Second Takes
Andrea Thorn
Published 28 February 2023
-
![]()
Radical reactions
Yvain Nicolet
Published 31 January 2023
![]()
Floppy Physics
Eva Nogales
Published 30 November 2022
-
![]()
Structure of Equity
Jamaine Davis
Published 28 September 2022
![]()
Life and Death of a Cell
Evris Gavathiotis
Published 28 July 2022
-
![]()
Follow the glow
Kurt Krause
Published 29 April 2022
![]()
Resolution solutions
Willy Wriggers
Published 25 February 2022
-
![]()
Of enzymes and membranes
Ming Zhou
Published 28 October 2021
![]()
Step-by-step
Gabrielle Rudenko
Published 26 September 2021
-
![]()
Moving muscle
Montserrat Samso
Published 26 July 2021
![]()
Particle catcher
Stefan Raunser
Published 28 June 2021
-
![]()
Designer drugs
Ho Leung Ng
Published 25 February 2021
![]()
Right place, right time
Ernesto Fuentes
Published 29 January 2021
-
![]()
Shape-shifting secrets of membranes
James Hurley
Published 27 November 2020
![]()
Enzymatic action
Cynthia Wolberger
Published 28 September 2020
-
![]()
Rules of motion
Priyamvada Acharya
Published 31 July 2020
![]()
Cosmic Squared
Michael Cianfrocco
Published 27 June 2020
-
![]()
Kaps are Cool
Yuh Min Chook
Published 28 April 2020
![]()
Spiraling into focus
Carsten Sachse
Published 29 March 2020
-
![]()
Seeing cilia
Alan Brown
Published 27 February 2020
![]()
For the Love of EM
Guy Schoehn
Published 27 January 2020
-
![]()
Protein Puddles
Michael Rosen
Published 16 December 2019
![]()
Changing channels
Daniel Minor Jr.
Published 27 September 2019
-
![]()
Listening Tips
Marcos Sotomayor
Published 30 July 2019
![]()
Beyond Cool
Published 31 May 2019
-
![]()
Hao Wu
A Higher Order
Published 30 May 2019
![]()
Aye Aye Captain
Alexandre Bonvin
Published 29 April 2019
-
![]()
The PARP Family Family
John Pascal
Published 28 February 2019
![]()
Frame by frame
Nikolaus Grigorieff
Published 28 January 2019
-
![]()
Predicting Success
Bil Clemons
Published 18 December 2018
![]()
Curiouser and Curiouser
Ramaswamy Subramanian
Published 27 November 2018
-
![]()
Rely on This
Sjors Scheres
Published 26 October 2018
![]()
Proteins out of bounds
Gerhard Wagner
Published 27 September 2018
-
![]()
Hiding in plain sight
Gaya Amarasinghe
Published 27 July 2018
![]()
Jumping Genes
Orsolya Barabas
Published 27 June 2018
-
![]()
Data Whisperer
Karolin Luger
Published 30 May 2018
![]()
Flipping the Switch
Jacqueline Cherfils
Published 27 April 2018
-
![]()
Tooling Around
Andrew Kruse
Published 29 March 2018
![]()
Comings and Goings
Tom Rapoport, Ph.D.
Published 23 February 2018
-
![]()
Transcriptional Rhythm
Seth Darst
Published 27 January 2018
![]()
The Language of Gene Regulation
Daniel Panne
Published 21 November 2017
-
![]()
Not Your Average Protein
James Fraser
Published 23 October 2017
![]()
Message Received
Sebastien Granier
Published 24 August 2017
-
![]()
Resistance is Futile
Celia Schiffer
Published 28 July 2017
![]()
Twist of Fate
Leemor Joshua-Tor
Published 28 June 2017
-
![]()
Drug Designer
John Buolamwini
Published 30 May 2017
![]()
Mathematically Minded
James Holton
Published 28 April 2017
-
![]()
Garbage Out
Kay Diederichs
Published 30 March 2017
![]()
Fixer Upper
Brandt Eichman
Published 27 February 2017
-
![]()
Mobilizers
Phoebe Rice
Published 31 January 2017
![]()
Escape Artist
Katya Heldwein
Published 19 December 2016
-
![]()
Nature’s Confectioner
Jochen Zimmer
Published 29 November 2016
![]()
State of Fusion
Jason McLellan
Published 27 October 2016
-
![]()
Here Be Dragons
Brian Fox
Published 28 September 2016
![]()
SBGrid Assumes Ownership of PyMOLWiki
Published 15 September 2016
-
![]()
Pharm Team
Oleg Tsodikov
Published 24 August 2016
![]()
Spiro-Gyra
Alejandro Buschiazzo
Published 27 July 2016
-
![]()
Turning the DIALS
Nicholas Sauter
Published 29 June 2016
![]()
Pipeline Dreams
Bridget Carragher and Clint Potter
Published 26 April 2016
-
![]()
U-Store-It
The SBGrid Data Bank provides an affordable and sustainable way to preserve and share structural biology data
Published 28 March 2016
![]()
Big Questions, Big Answers
Jennifer Doudna
Published 22 February 2016
-
![]()
Not a Structural Biologist
Enrico Di Cera
Published 17 December 2015
![]()
Divide and Conquer
Kevin Corbett
Published 19 November 2015
-
![]()
Computing Cellular Clockworks
Klaus Schulten
Published 23 October 2015
![]()
Trans-Plant
Gang Dong
Published 26 September 2015
-
![]()
Keep on Moving
James Berger
Published 23 August 2015
![]()
Totally Tubular
Antonina Roll-Mecak
Published 27 July 2015
-
![]()
From Disorder, Function
Julie Forman-Kay
Published 29 June 2015
![]()
Into Alignment
Geoff Barton
Published 27 May 2015
-
![]()
Two Labs, Many Methods
Michael Sattler
Published 28 April 2015
![]()
Picture This
Georgios Skiniotis
Published 20 March 2015
-
![]()
Intron Intrigue
Navtej Toor
Published 20 February 2015
![]()
Cut and Paste
Martin Jinek
Published 28 January 2015
-
![]()
Basics and Beyond
Qing Fan
Published 18 December 2014
![]()
Bloodletting and Other Studies
Pedro José Barbosa Pereira
Published 25 November 2014
-
![]()
Wire Models, Wired
A brief history of UCSF Chimera
Published 29 October 2014
![]()
In Search of…New Drugs
Doug Daniels
Published 30 September 2014
-
![]()
An Affinity for Affinity…and Corals
John C. Williams
Published 29 August 2014
![]()
Pete Meyer, Ph.D.
Research Computing Specialist
Published 22 August 2014
-
![]()
Justin O'Connor
Sr. System Administrator
Published 20 August 2014
![]()
Carol Herre
Software Release Engineer
Published 15 August 2014
-
![]()
Elizabeth Dougherty
Science Writer
Published 13 August 2014
![]()
Andrew Morin, Ph.D.
Policy Research Fellow
Published 11 August 2014
-
![]()
Jason Key, Ph.D.
Associate Director of Technology and Innovation
Published 8 August 2014
![]()
Piotr Sliz, Ph.D.
Principal Investigator, SBGrid
Published 1 August 2014
-
![]()
New Kid on the Block
James Chen
Published 29 July 2014
![]()
Membrane Master
Tamir Gonen
Published 30 June 2014
-
![]()
The Natural Bridge
Piotr Sliz
Published 13 June 2014
![]()
Surprise, Surprise
Catherine Drennan
Published 26 April 2014
-
![]()
Gone Viral
Olve Peersen
Published 20 March 2014
![]()
All Who Wander Are Not Lost
Frank Delaglio
Published 24 February 2014
-
![]()
The Raw and the Cooked
Graeme Winter
Published 24 January 2014
![]()
Vacc-elerator
Peter Kwong
Published 17 December 2013
-
![]()
Structural Storyteller
Karin Reinisch
Published 15 November 2013
![]()
The Fixer
Jane Richardson
Published 28 October 2013
-
![]()
Inside the Box
Mishtu Dey
Published 17 September 2013
![]()
Sensing a Change
Brian Crane
Published 16 August 2013
-
![]()
Towards Personalized Oncology
Mark Lemmon
Published 16 July 2013
![]()
Brush with Fame
Yizhi Jane Tao
Published 14 June 2013
-
![]()
Toxic Avenger
Borden Lacy
Published 21 May 2013
![]()
Pushing the Boundaries
Stephen Harrison
Published 22 April 2013
-
![]()
Strength in Numbers
Joseph Ho
Published 18 March 2013
![]()
One Lab, Many Methods
Wesley Sundquist
Published 12 February 2013
-
![]()
Unplanned Pioneer
Tim Stevens
Published 15 January 2013
![]()
From Actin to Action
Emil Pai
Published 11 January 2013
-
![]()
Unstructured
A Brief History of CCP4
Published 12 December 2012
![]()
Stop, Collaborate and Listen
Eleanor Dodson
Published 5 November 2012
-
![]()
X-PLORer
Axel Brunger
Published 1 October 2012
![]()
Share the Wealth
Zbyszek Otwinowski
Published 22 August 2012
-
![]()
Unraveling RNA
Anna Pyle
Published 18 July 2012
![]()
Sharper Image
Pawel Penczek and SPARX
Published 4 June 2012
-
![]()
Creative Copy Cat
Pamela Bjorkman
Published 25 April 2012
![]()
Charm and Diplomacy
Gerard Kleywegt
Published 7 March 2012
-
![]()
From Curiosity to Cure
Marc Kvansakul
Published 13 December 2011
![]()
The Lure of the Sandbox
Paul Emsley and Coot
Published 15 October 2011
-
![]()
Springsteen, Tolkien, Protein
Alwyn Jones and Frodo
Published 17 June 2011
![]()
Structures Solved Simply
Paul Adams and Tom Terwilliger on Phenix
Published 2 June 2011
-
![]()
Playing the Odds
Randy Read and Phaser
Published 19 May 2011
![]()
Escape from the Darkroom
Wolfgang Kabsch and XDS
Published 19 May 2011
-
![]()
Better, Faster, Stronger, More
Victor Lamzin and ARP/wARP
Published 17 May 2011
![]()
Crystallography for Kids
Lynne Howell
Published 17 May 2011





































































































































