The Final Phase
George Phillips
Rice University
Published May 31, 2025
For a person intent on tiny forms, George Phillips's career has been closely tied to big initiatives that have added new categories of structures to the Protein Data Bank. He has pioneered dynamic crystallography studies that reveal dramatic split-second moments of molecular activity. And his team has animated the atomic handbook on myoglobin, the well-studied molecular oxygen reservoir found in muscles.
But his most-cited paper remains a side note to the major themes of his laboratory. It happened almost by chance as a young faculty member at Rice University in Houston, Texas.
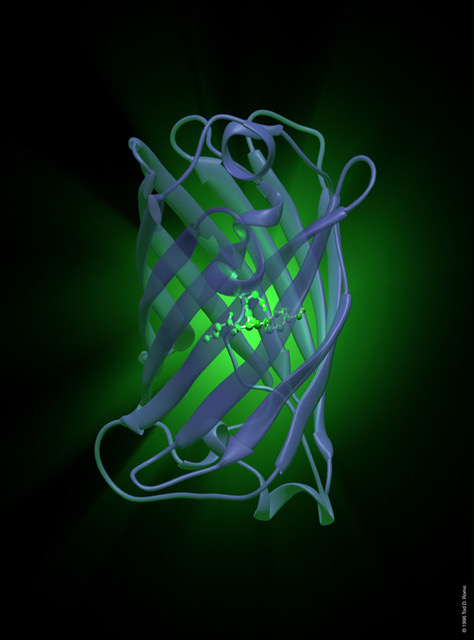
"That was actually kind of a one-off thing," Phillips says. "One of my college roommates called me and said, 'Hey, nobody's done the structure of green fluorescent protein. Do you want to work on it?' It crystallized easily, and we saw the structure of the native protein."
It was a cylinder shape, a beta sheet wrapped around an alpha helix. "We called it the beta-can," he says. "It was like Popeye's can of spinach." (Nature Biotechnology, 1996)
Fast-forward three decades, and Phillips may be one of the few US researchers in early 2025 whose future is not in jeopardy from the threat of massive cuts in federal funding that are looming over the US biomedical infrastructure and becoming a harsh reality for many. That's because Phillips is about to retire. Again.
Phillips earned his PhD at Rice and returned to start a new lab after a stint at the University of Illinois-Urbana Champaign. Then he spent a dozen years at University of Wisconsin-Madison, leaving with professor emeritus status when he returned to Rice in 2012.
Now Phillips is in the last few months of running his Rice lab, a decision made three years ago. He'll keep his office for a final foundation-funded project to tinker with a new way to solve the "phase problem" that bedevils X-ray crystallography.
Phase information is crucial to reconstruct the arrangements of atoms in a molecule, but it cannot be measured in the diffraction waves from a crystal, and so it is missing from every experimental data set. It's a problem that needs to be solved to visualize any molecule analyzed by X-ray crystallography.
"The classical methods take a lot of skilled crystallographer time, and we want to see if we can help that process along by training a neural network to shortcut the problem," he says.
The computational approach was inspired by the way AlphaFold cracked the protein folding problem. He, colleague Anastasios Kyrillidis and graduate student Tom Pan showed proof of concept on simple dipeptides and have since worked their way up from two amino acids to tackle molecules composed of 50–150 amino acids.
The project highlights a theme underlying Phillips's life in science. He seeks to make the technical chores more efficient, so that structural biologists and crystallographers can "spend more of their brain power to find interesting problems or think about what they mean," he says.
He views each of the two sub-specialties as the antidote for the other when things get boring. "A structural biologist, even if they're using the technique of crystallography, is primarily motivated by getting the structure and explaining what the structure means. Crystallographers are motivated by understanding the science of crystallography and advancing the art of crystallography for its own sake … and are mostly agnostic about what they're studying."
In his crystallography role, he has helped lead federally funded long-term projects that have advanced the tools and techniques for structural biology.

Unknown Proteins
In one, Phillips and his UWM colleagues won funding to establish one of the centers of the Protein Structure Initiative. The initiative was part of an international effort in structural proteomics.
"Structural biologists had looked around, and the structures that were being solved were things that had been studied in the lab already, and one wanted a reductionist explanation of how they worked," he says. "The [new] idea was, instead of going after the known proteins, let's go after the 'unknowneome,' we called it, tongue in cheek."
Combining expertise in X-ray crystallography (Phillips), nuclear magnetic resonance (John Markley) and Arabidopsis transcriptomics (Mike Sussman), the center took on the only eukaryotic organism in the initiative. Their charge was to go after structures of proteins with unknown functions and to automate the procedures for efficient high-throughput crystallography.
The larger initiative churned out more than 600 experimental structures every year for 10 years, assisted by new software, cloning procedures, and high throughput protein crystallization robots. Phillips's main responsibility at the UW center was "to oversee the crystallographic operation, everything from quality control on the protein through the crystallization, the screening of the crystals, the collecting of data at the synchrotron, the solving of the structure and the publishing of the work," he says.
Many times, initiative researchers found, even when an amino acid sequence looked like nothing anyone had seen, the protein structure wound up resembling a known protein. That led to the possibility of a kind of reverse structural biology, where researchers could test if the unknown doppelgänger had a similar biological function. In the last few years of the initiative, Phillips and other research partners put these new technologies to use in the discovery of novel enzymes found in natural product pathways to explore their pharmaceutical potential.
Laser! Detector! Action!
In another initiative just winding down now, Phillips teamed up with a consortium working on the next generation of structural biology - to capture molecules in motion, rather than the usual static snapshots. Launched in 2013, the BioXFEL Science and Technology Center featured the first X-ray free-electron laser (XFEL) on the planet based at Stanford University's linear accelerator facility. It was about a billion times brighter than other sources. Since then, a half dozen more such state-of-the-art lasers in facilities around the world have been set up, he says.
They took an approach called time-resolved crystallography to the next level. One of Phillips's jobs was to develop the right crystals for the system and to connect the results to meaningful biological questions. Other team members focused on how to design jets to shoot a focused stream of crystals and how to process the diffraction data.
"It takes a lot of protein to do these experiments," he notes. "An X-ray free electron laser produces rays that are so intense that your crystal is vaporized more or less instantly," Phillips says. "It's called serial femtosecond crystallography - serial, because you have to have a fresh crystal each time, and femtosecond, because that's the length of the pulse."
In BioXFEL, Phillips also helped pioneer another technique of catching split-second conformational changes within crystals. Many enzymes remain active in crystal samples and may have room to wiggle if no wild motions are required. Each batch of crystals can be mixed with a molecular partner for different periods of time before being streamed and zapped. "With different time delays, you take your crystallographic data, and then you can solve the structures of steps along the way, and then play them like a movie," he says.
Using this technique, for example, Phillips and his colleagues studied an enzyme produced by bacteria to defend themselves against antibiotics. Thanks to XFEL, they watched the enzyme bind to the antibiotic and saw the chemical changes as the enzyme degraded it. (Structural Dynamics, 2016; Nature Communications, 2023)
New ways of advancing scientific knowledge are great, but the consortium projects also satisfied the educator in Phillips. Early in his first Rice tenure, he wrote an online program for teaching crystallography basics, called X-RayView (https://xrayview.org/). It now also works on smartphones. For BioXFEL, he rebooted the educational component, serving as education and diversity director for eight years, which included hosting undergraduate students with diverse backgrounds in a summer research opportunity program.
He believes in giving back to the scientific community in other ways. He is editor-in-chief of the journal Structural Dynamics and past president of the American Crystallographic Association.
Early Days
Phillips was born in Dallas, the son of an airline pilot (father) and flight attendant (mother) who resigned as required when they married. His dad had been a transport pilot in World War II and the Korean War. The family relocated to Marin County, near San Francisco, where his dad worked for an airline chartered to fly US troops and supplies back and forth to Vietnam.
Science and math came easily to Phillips in high school in California. He returned to Texas for college at Rice in Houston. At a chemistry department open house for undergraduates, he wandered into a lab where students and postdocs were building atomic models. They were stacking plastic sheets to put together a three-dimensional representation of a chemical compound. This was before computer graphics, and it looked like they were playing with Tinker Toys.
They told him it was called crystallography, and it was kind of like solving puzzles to learn more about the chemical compound. "Wait. You can get paid to solve puzzles and play with Tinker Toys? I was all over that," says Phillips, whose office is decorated with modern colorful 3D printed plastic molecular models.
When he was a senior, Phillips came face-to-face with protein crystallography for the first time. A biochemistry professor, Florante Quiocho, had just grown some crystals of a bacterial binding protein and badly wanted a student to solve the structure. He convinced Phillips to leapfrog his pending undergraduate graduation and simultaneously begin his PhD project. He did a postdoctoral fellowship at University of Illinois, where he studied muscle regulatory proteins.
Animating myoglobin
Soon after he started his first lab at Rice, Phillips began what became a long collaboration with colleague John Olson, who was studying myoglobin, an oxygen-storing protein found in muscles, as a model for hemoglobin, the oxygen-transporting protein in blood.
Myoglobin was the first protein to have its three-dimensional structure solved in 1958. The static structure revealed the oxygen was trapped and it needed to move to function. Several generations of scientists since then used available techniques to explore potential conformational changes. Controversy ensued about how oxygen gets in and out.
"I did a lot of work with John on myoglobin trying to understand how the oxygen and other ligands get in and out of the protein," Phillips says. Long before BioXFEL, he came up with the idea of making a movie to see the action. His team used a orange laser beam to knock carbon monoxide (an experimental surrogate for oxygen) off the iron atom and watch where it went. "It rattled around and stuck again," he says. But that rattle also triggered some other movements that led the team to suggest a histidine gate may open as a part of its function. (Science, 2003)
A very stable version of myoglobin enables whales and other deep-diving mammals to produce high concentrations of the protein before it acquires the oxygen, the team discovered more recently. As a result, whales can pack 10–20 times more myoglobin into their muscles than humans and explains why their meat is so dark.
As he winds down his lab work, Phillips is turning his administrative and technical skills to a holistic garden plot at Rice. Students provide the labor, and they get to eat the fruits of their farming at university food services. He looks forward to tilling soil, pulling weeds, and maybe even fixing the tractor. In the meantime, he's using a lower-tech laser to etch paving bricks with donor names as a fundraiser for the garden. "It's very relaxing."
-Carol Cruzan Morton




































































































































